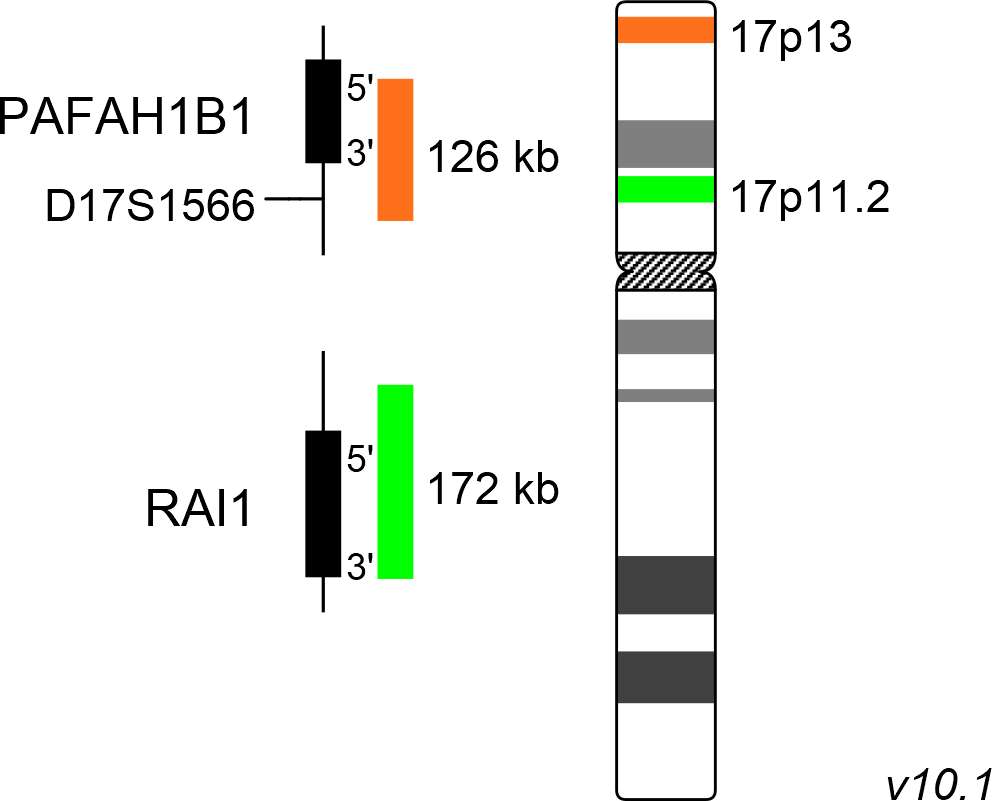
First described in 1982 by Ann C. M. Smith and her colleagues, Smith-Magenis syndrome (SMS) has an estimated prevalence of 1:25.000 newborns. While interstitial deletions in the segment 17p11.2 are the genetic basis of Smith-Magenis syndrome (SMS), deletions of the more distal part 17p13.3 lead to Miller-Dieker syndrome (MDS). SMS is characterized by relatively mild craniofacial and skeletal abnormalities, delay in psycho-motoric development, and specific behavioural disorders. In addition to neuro-behavioural problems, often including self-injurious pattern, SMS patients suffer from a disturbed sleep cycle, as the day and night melatonin secretion cycle is reversed.
MDS patients show a neuro-developmental and structural disorder of the brain, called lissencephaly. The typical walnut structure of the brain containing fissures and gyri is hardly recognizable. Craniofacial dysmorphism, decreased head circumference, delay in psychomotor development and seizures are further symptoms of this syndrome. The SMS inducing deletions of 17p11.2 have no preferential breakpoints, but most patients analyzed show deletions smaller than 4Mb. The key gene responsible for the manifestation of the clinical symptoms of SMS is RAI1 (retinoic acid induced 1), coding for a transcription factor. One of the crucial genes involved in the development of Miller-Dieker lissencephaly and craniofacial dysmorphism is the PAFAH1B1 gene encoding the regulatory subunit of the platelet activating factor acetylhydrolase 1b.
Clinical Applications
- Microdeletion Syndrome (MicroDel)