XL 20q12/20qter plus
Deletion/Isochromosome Probe
- Order Number
- D-5121-100-OG
- Package Size
- 100 µl (10 Tests)
- Chromosome
- 2020
- Regulatory Status
- IVDD
IVDR Certification
This probe is IVDR-certified in compliance with the Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR).
MetaSystems Probes has already certified a wide range of FISH probes, according to IVDR.
This product remains IVDD-certified until further notice.
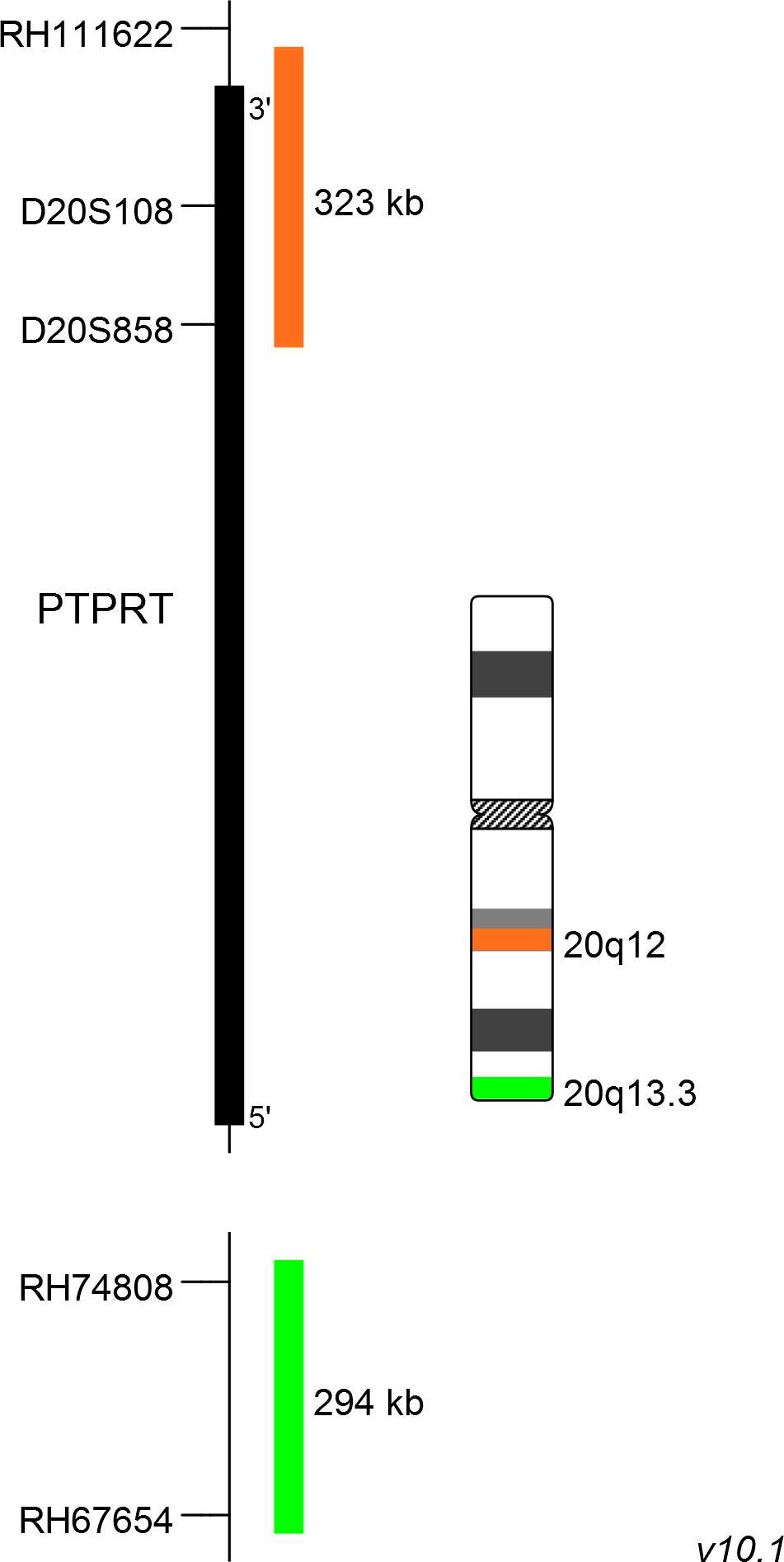
XL 20q12/20qter plus consists of an orange-labeled probe hybridizing to the PTPRT gene region at 20q12 and a green-labeled probe hybridizing to a region at 20q13.3.
Probe maps for selected products have been updated. These updates ensure a consistent presentation of all gaps larger than 10 kb including adjustments to markers, genes, and related elements. This update does not affect the device characteristics or product composition. Please refer to the list to find out which products now include updated probe maps.
Probe map details are based on UCSC Genome Browser GRCh37/hg19, with map components not to scale.
Myelodysplastic syndromes (MDS) are a group of hematopoietic stem cell disorders associated with ineffective hematopoiesis and peripheral blood cytopenias. Approximately 40% of MDS cases are progressing to acute myeloid leukemia. In about 50% of de novo MDS, cytogenetic aberrations are observed; deletions are predominant, translocations are rare. Recurrent abnormalities are del(5q), monsomy 7, del(7q), del(20q), del(17p) and del(11q).
A chromosome 20q deletion is seen in about 2% of MDS cases. Patients with a sole del(20q) have a favourable outcome compared to patients with additional abnormalities such as del(5q), del(7q), monosomy 7 and trisomy 8. The majority of patients with del(20q) have an interstitial deletion between 20q11.2 and 20q13.3. In rare cases, the 20q deletion can occur as an isoderivative chromosome ider(20q) with loss of the p-arm of chromosome 20 and partial trisomy of the remaining regions on the q-arm.
Clinical Applications
- Myelodysplastic Syndrome (MDS)
- Acute Myelogenous Leukemia (AML)

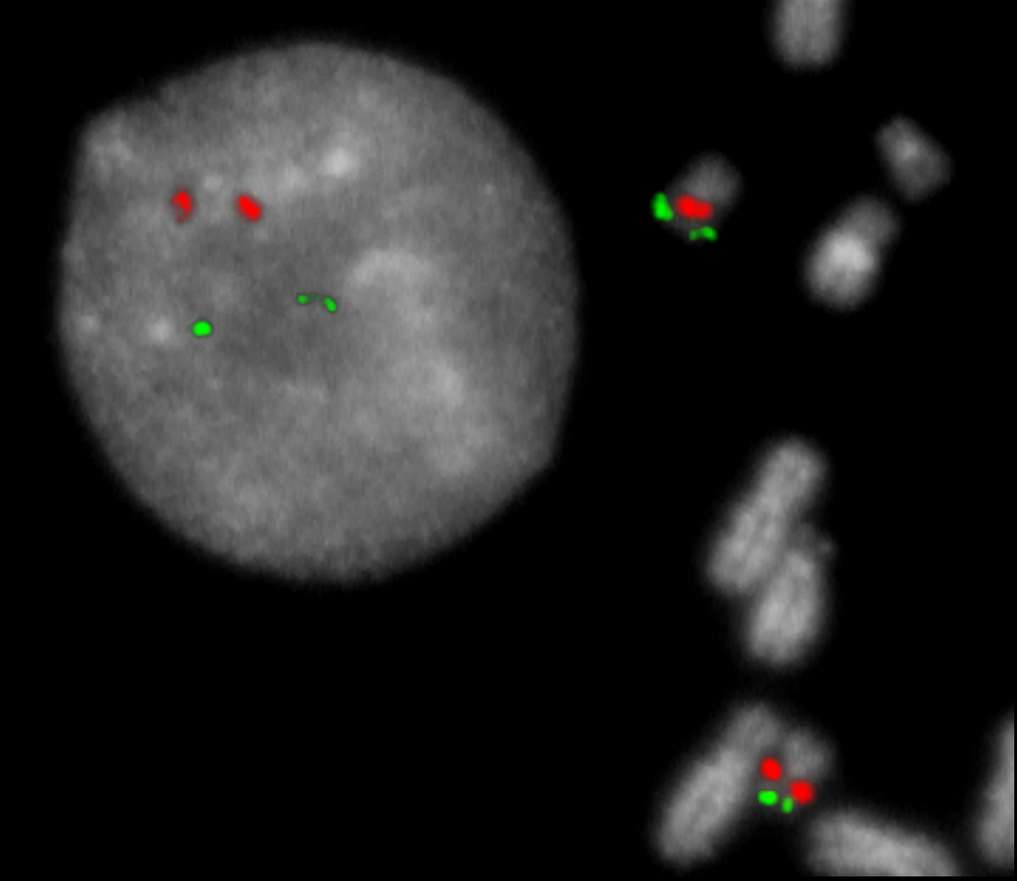
Normal Cell:
Two green (2G) and two orange (2O) signals.

Aberrant Cell (typical results):
Two green (2G) and one orange (1O) signal resulting from loss of one orange signal.

Aberrant Cell (typical results):
Three green (3G) and one orange (1O) signal resulting from the presence of ider(20q).
- Kurtin et al (1996) Am J Clin Pathol 106:680-688
- Douet-Gilbert et al (2008) Br J Haematol 143:716-720
- Bacher et al (2014) Br J Haematol 164:822-833
Certificate of Analysis (CoA)
or go to CoA Database