Probes Newsletter 03/2020
Newsletter 03/2020
XCyting new KMT2A dual fusion probes…
We are very pleased to announce 5 new innovations in our locus-specific probes product line for Hematology. The new probes are designed to detect common chromosomal rearrangements with KMT2A (formerly MLL) gene involvement in the context of acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). As therapeutic strategies and prognosis are tightly linked to the respective kind of KMT2A rearrangement, the reliable and rapid detection of these aberrations via FISH is of greatest interest. AFF1, MLLT3, MLLT1, MLLT10, ELL and AFDN are the most common translocation partners in KMT2A associated leukemia cases. The now completed portfolio of KMT2A dual fusion probes covers translocations involving these six most frequent translocation partner genes and completes the already launched XL t(9;11) MLLT3/KMT2A DF probe: XL t(4;11) AFF1/KMT2A DF, XL t(11;19) KMT2A/MLLT1 DF, XL t(10;11) MLLT10/KMT2A DF, XL t(11;19) KMT2A/ELL DF and XL t(6;11) AFDN/KMT2A DF. In addition, our KMT2A break apart probes XL KMT2A BA and XL MLL plus complement this newly established product portfolio. For further product information, please visit www.metasystems-probes.com.
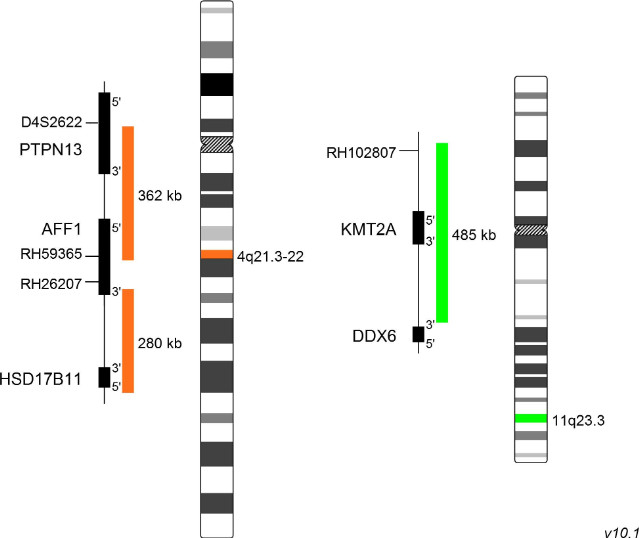
XL t(4;11) AFF1/KMT2A DF
Clinical Applications: AML/ALL
The fusion of KMT2A and AFF1 (AF4/FMR2 family member 1) is the most frequent KMT2A translocation observed in ALL cases. About 60% of ALL patients carrying KMT2A rearrangements are characterized by the KMT2A-AFF1 fusion gene. The breakpoint distribution in the KMT2A gene shows an age dependent switch. Infant patients mainly have breakpoints in intron 11 of KMT2A, whereas adult patients have a tendency for breakpoints within intron 9. Oncogenic KMT2A-AFF1 fusions have been implicated in the DOT1L and SEC transcriptional complexes. AFF1 itself plays an important role in the regulation of RNA polymerase II dependent transcription, elongation and chromatin remodeling mediated by histone methylation. AFF1 is the docking platform for several components of the DOT1L complex consisting of DOT1L, a H3K79 methyltransferase, MLLT3, MLLT1, and MLLT10, whose genes are also common translocation partners of KMT2A. Mouse studies revealed increased and extended histone methylation signatures in the presence of KMT2A-AFF1 and reciprocal AFF1-KMT2A fusions. Therefore, DOT1L inhibitors are promising candidates for clinical treatment which are currently being evaluated.
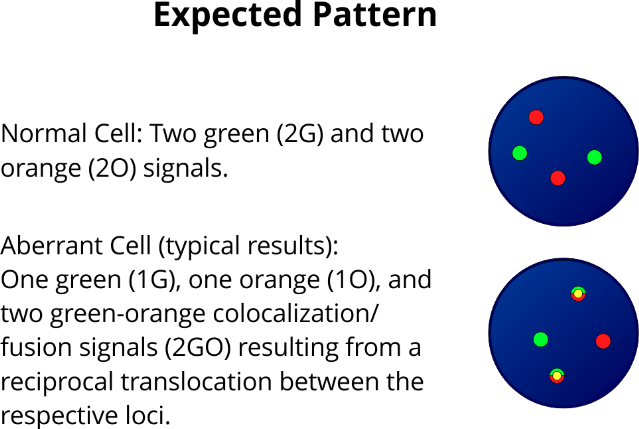
XL t(4;11) AFF1/KMT2A DF is designed as a dual fusion probe. The orange labeled probe hybridizes to the AFF1 gene region at 4q21, the green labeled probe hybridizes to the KMT2A gene region at 11q23.
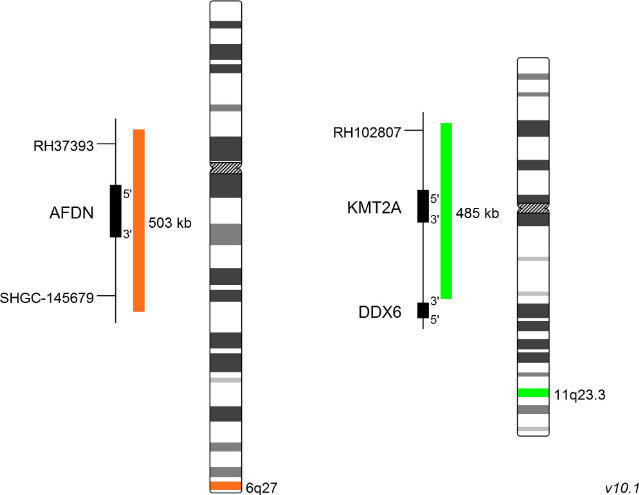
XL t(6;11) AFDN/KMT2A DF
Clinical Applications: AML/ALL
The AFDN (afadin) gene, formerly known as MLLT4 and AF6, is located on chromosome 6 (6q27). KMT2A-AFDN fusions result from translocations of the type t(6;11)(q27;q23). The most frequent breakpoint in the KMT2A gene leading to this translocation is positioned in intron 9. The majority of ALL patients have breakpoints in exon 1 or 2 of the AFDN gene. Intriguingly, T-cell ALL patients show a significantly higher percentage of KMT2A-AFDN and KMT2A-MLLT1 fusions than other ALL subgroup patients. However, within the cohort of patients with KMT2A-AFDN rearrangements, unusual KMT2A breakpoints in intron 21 and 23 have been detected, in a minority of cases. One cellular consequence of the KMT2A-AFDN fusion is an increased and extended H3K79 methylation signature, which is also being observed in the presence of KMT2A-AFF1, AFF1-KMT2A, KMT2A-MLLT3, KMT2A-MLLT1, and KMT2A-MLLT10 fusions. This increased methylation, a prerequisite for the maintenance of RNA transcription, is mediated by DOT1L, a histone H3K79 methyltransferase, which interacts with AFF1, MLLT1, MLLT3 and MLLT10, directly or indirectly. Therefore, DOT1L inhibitors are promising candidates for clinical treatment which are currently being evaluated.
XL t(6;11) AFDN/KMT2A DF is designed as a dual fusion probe. The orange labeled probe spans the breakpoint at 6q27 (AFDN), the green labeled probe spans the breakpoint at 11q23.3 (KMT2A).
XL t(10;11) MLLT10/KMT2A DF
Clinical Applications: AML/ALL
MLLT10 (MLLT10 Histone Lysine Methyltransferase DOT1L Cofactor), previously known as AF10, is one of the most frequent fusion partners of KMT2A across all acute leukemia cases. KMT2A-MLLT10 fusions result from multiple breakpoints in both gene loci. The subsequent chromosomal rearrangements include reciprocal translocations, insertions, inversions, deletions and duplications. MLLT10 is a cofactor of the histone H3K79 methyltransferase DOT1L and mediates the interaction of AFF1, MLLT1 and MLLT3 with DOT1L. The consequence of the presence of KMT2A-AFF1, AFF1-KMT2A, KMT2A-MLLT3, KMT2A-MLLT1, and KMT2A-MLLT10 fusions is an increased and extended H3K79 methylation signature that is a requirement for the maintenance of RNA transcription. DOT1L inhibitors are promising candidates for clinical treatment which are currently being evaluated.
XL t(10;11) MLLT10/KMT2A DF is designed as a dual fusion probe. The orange labeled probe spans the breakpoint at 10p12.3 (MLLT10), the green labeled probe spans the breakpoint at 11q23.3 (KMT2A).
XL t(11;19) KMT2A/ELL DF
Clinical Applications: AML/ALL
Fusions between KMT2A and ELL (elongation factor for RNA polymerase II), caused by translocations of the type t(11;19)(q23;p13.1), belong to the most common KMT2A fusion genes in AML. Approximately 11% of AML patients carrying KMT2A rearrangements are characterized by the KMT2A-ELL fusion gene. The breakpoints within the KMT2A gene resulting in KMT2A-ELL fusions are found in intron 9 in the case of patients younger than one year and in intron 11 in the case of patients older than one year. This breakpoint distribution is unique among all KMT2A fusions. The outcome of patients with breakpoints in KMT2A intron 11 is worse compared to patients with upstream breakpoints. ELL is a component of the super elongation complex (SEC). Chimeric KMT2A-ELL fusion proteins have the ability to recruit SEC resulting in aberrant gene expression.
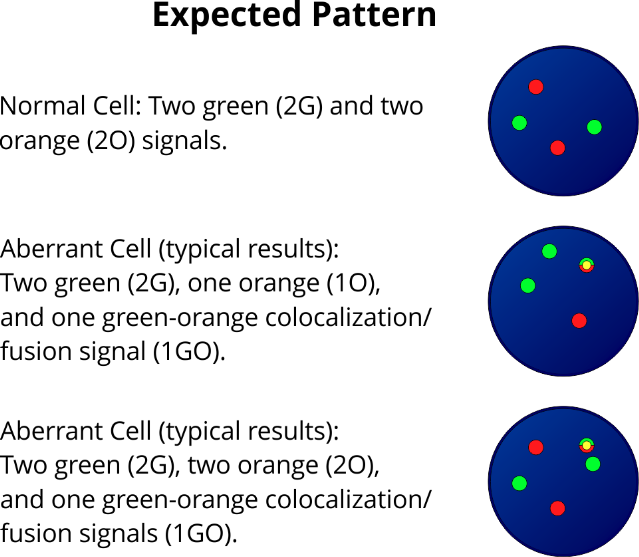
XL t(11;19) KMT2A/ELL DF is designed as a dual fusion probe. The orange labeled probe is located in band 19p13.1 (ELL), the green
labeled probe spans the breakpoint at 11q23.3 (KMT2A).
XL t(11;19) KMT2A/MLLT1 DF
Clinical Applications: AML/ALL
MLLT1 (MLLT1 super elongation complex subunit), originally designated as ENL, is one of the most common KMT2A fusion partners. KMT2A-MLLT1 fusions result from translocations of the type t(11;19)(q23;p13.3). Most patients carrying a KMT2A-MLLT1 fusion have breakpoints in intron 11 of the KMT2A gene. KMT2A-MLLT1 fusions are prevalent in both AML and ALL. Multiple KMT2A translocation partner genes, including MLLT1, are organized within the DOT1L transcriptional complex. AFF1 serves as DOT1L complex docking platform for MLLT1 and MLLT3, while MLLT10 directly mediates this interaction. More precisely, the MLLT1 protein has been shown to interact with AFF1 via its C-terminus. Due to the close interaction of these KMT2A fusion partners with DOT1L, DOT1L inhibitors are considered as promising candidates for treatment of such leukemia cases.
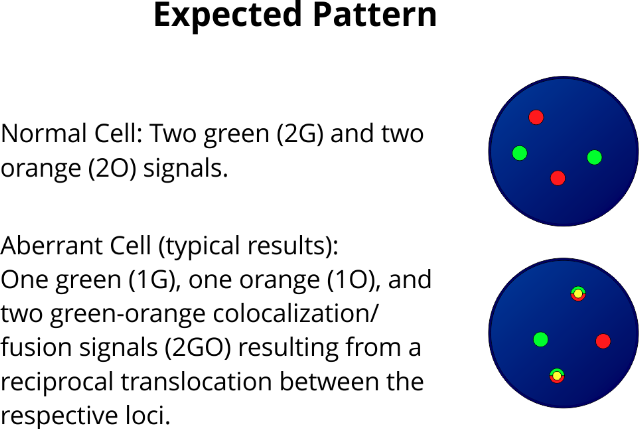
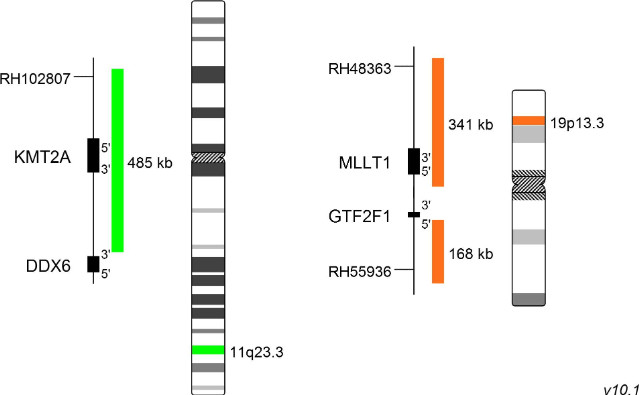
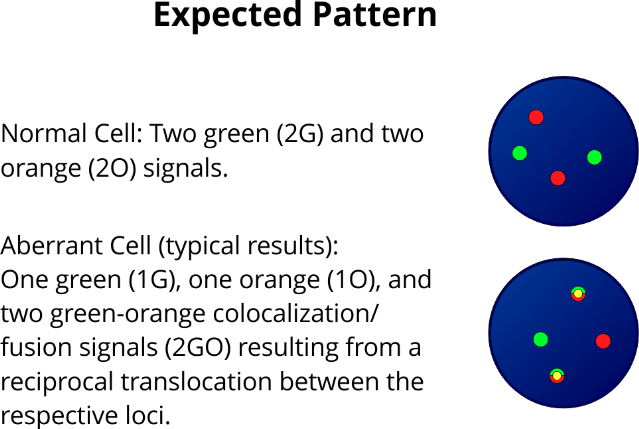
XL t(11;19) KMT2A/MLLT1 DF is designed as a dual fusion probe. The orange labeled probe is located in band 19p13.3 (MLLT1), the green labeled probe spans the breakpoint at 11q23.3 (KMT2A).