XA AneuScore II
Aneusomy Probe
- Order Number
- D-5609-500-TC
- Package Size
- (2x 50 Tests)
- Regulatory Status
- IVDR
IVDR Certification

This probe is IVDR-certified in compliance with the Regulation (EU) 2017/746 for in vitro diagnostic medical devices (IVDR).
MetaSystems Probes has already certified a wide range of FISH probes, according to IVDR.
Please use the switch to change to the IVDD product.
IVDDIVDRDiscover all IVDR-certified productsDownload Instructions For Use (IFU)
Until further notice, we will continue to provide IVDD products in various countries.
As part of our phased rollout, the IVDR-certified probes are now available in Australia, Austria, Belgium, Croatia, Cyprus, Czech Republic, Estonia, France, Germany, Iceland, Italy, Kuwait, Lebanon, Liechtenstein, Luxembourg, Malta, Netherlands, New Zealand, Poland, Slovakia, Slovenia, South Africa, and Switzerland, with the list of countries continuously expanding.
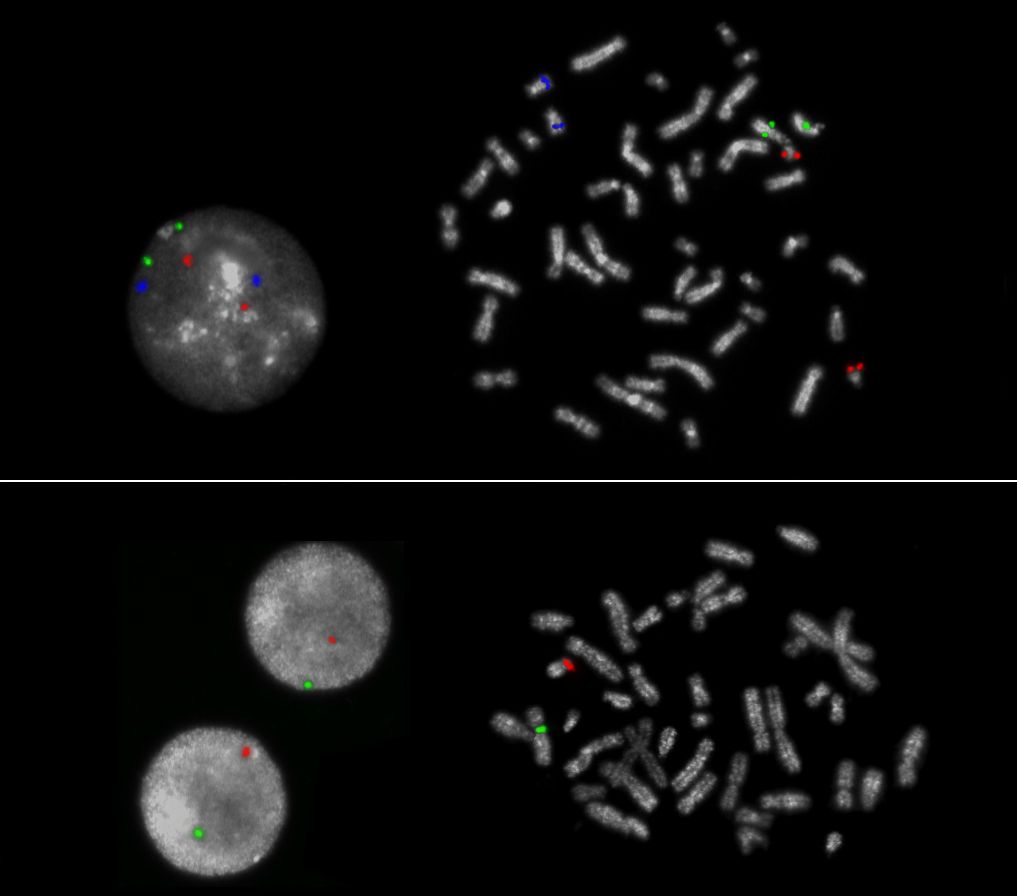
The XA AneuScore II contains different probe mixes provided in separate test vials.
5x XA 13/18/21 (D-5607-100-TC):
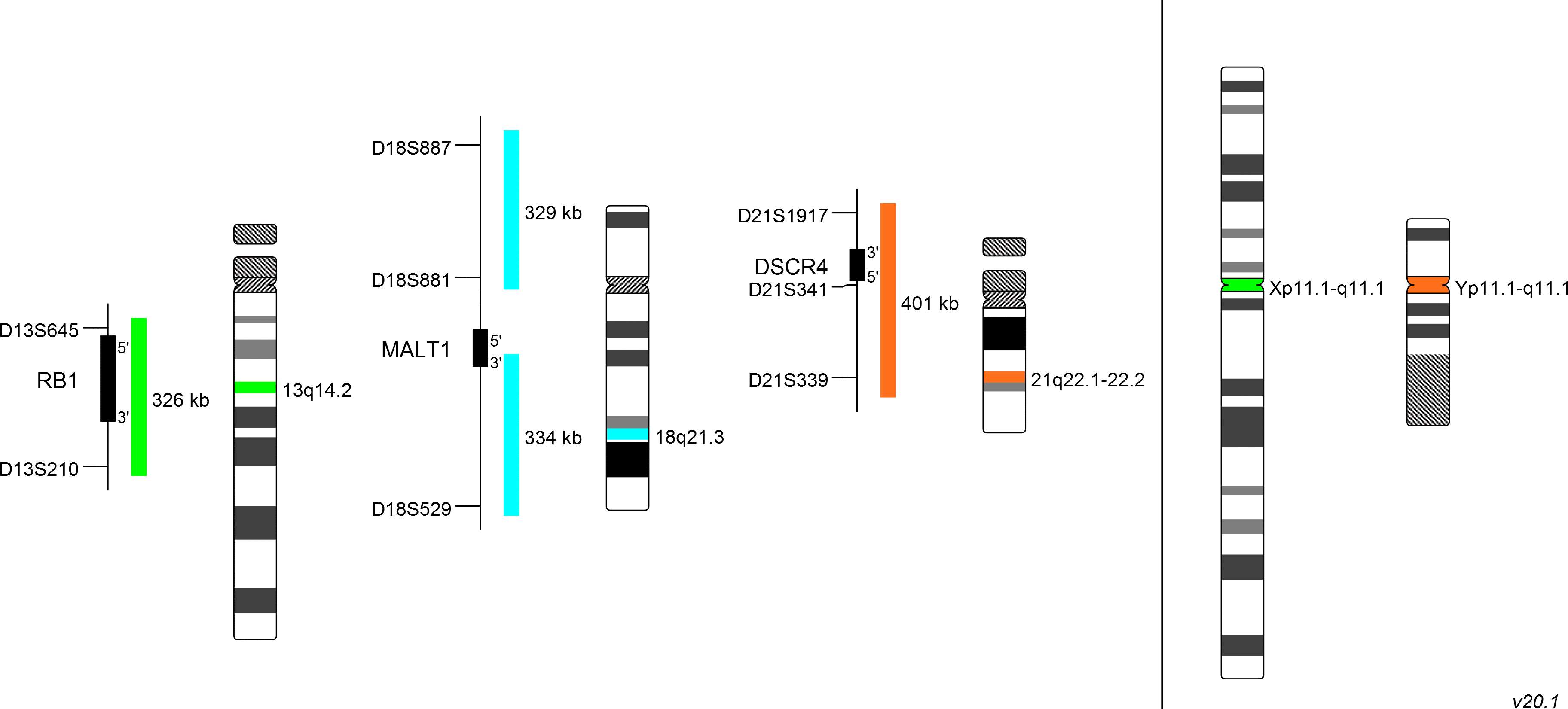
The XA 13/18/21 enumeration probe consists of a green-labeled probe hybridizing to the RB1 gene region at 13q14.2, an aqua-labeled probe hybridizing to the MALT1 gene region at 18q21.3 and an orange-labeled probe hybridizing to the DSCR4 gene region at 21q22.1-22.2.
5x XA X/Y (D-5608-100-OG):
The XA X/Y enumeration probe consists of a green-labeled probe hybridizing to the centromere of the X-chromosome and an orange-labeled probe hybridizing to the centromere of the Y-chromosome.
Probe maps for selected products have been updated. These updates ensure a consistent presentation of all gaps larger than 10 kb including adjustments to markers, genes, and related elements. This update does not affect the device characteristics or product composition. Please refer to the list to find out which products now include updated probe maps.
Probe map details are based on UCSC Genome Browser GRCh37/hg19, with map components not to scale.

For signal patterns please refer to: XA 13/18/21 (D-5607-100-TC) and XA X/Y (D-5608-100-OG).